With this article, you will learn:
- What is Bacillus subtilis as agricultural bio input
- How it protects crop
- How it nourishes crops
- How it improves soil
- What emerged from field trials
What is B. subtilis as agricultural bio input?
In the ever-evolving landscape of modern agriculture, the quest for sustainable, environmentally-friendly solutions has become paramount. One such solution that has gained significant attention in recent years is the application of beneficial microorganisms in agriculture. Among these microorganisms, B. Subtilis stands out as a promising player.
B. ubtilis, a non-pathogenic rod-shaped bacterium, has long been recognized for its versatility and adaptability. It is a ubiquitous inhabitant of soil, water, and the plant rhizosphere, due to its resistance to heat, drying, freezing temperatures and even disinfectants. (Vlamakis 2013).
- It is a natural soil bacterium that can help plants grow stronger and healthier.
- It produces substances that help plants fight off diseases and pests.
- It also helps plants take up nutrients from the soil more effectively.
- It also fosters soil health and mitigates environmental stresses.
As a result, B. subtilis can help to:
- Increase crop yields
- Improve soil quality
- Reduce the need for chemical fertilizers and pesticides
- Regenerate the soil
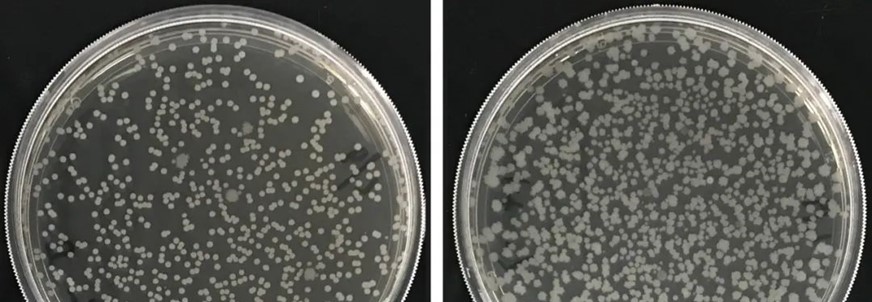
In the picture above, the two strains of B.Subtilis identified by Naturnova™ , MN776 and MN381. Two strains of B.Subtilis, MN776 and MN381, are excellent enzyme-producing and root-growing bacteria. When used together, they have a synergistic effect since they activate and support each other and grow better than when used alone. As a result, the compound of the two strains has a better growth-promoting and disease-preventing effect.
How does B. Subtilis protect the crops?
#
B.Subtilis is an outstanding colonizer with a doubling time of 20 minutes. it quickly occupies soil space and nutrients and prevents pathogens from invading (Errington, 2020)
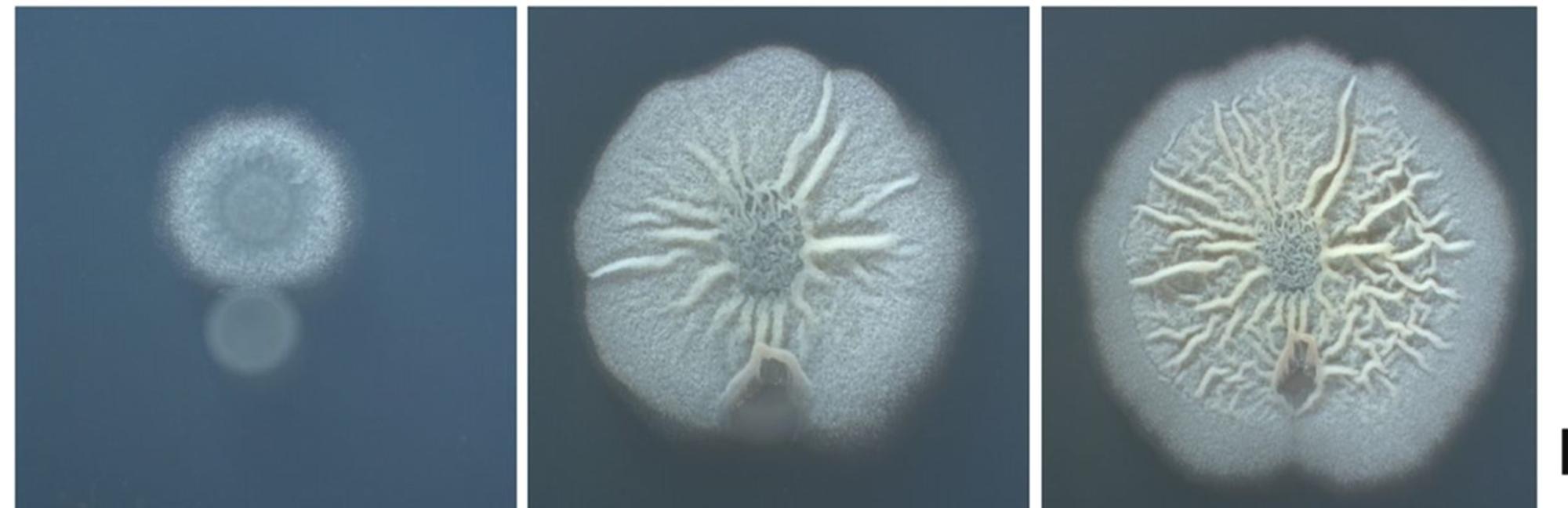
Picture 1: Development of B. Subtilis in agar plate, beginning (left), 48 H later (middle), 72 H later (right). Credits: Naturnova™ labs
Subtilis forms biofilms on plant roots thanks to special chemicals that help it stick, water channels, and chemical communication within the bacterial community (the biofilm). Biofilm formation is an adaptive response that allows bacteria to thrive in diverse environments and can have both beneficial and harmful effects in different contexts. As agricultural input, the biofilm becomes a protective shield against soil-borne pathogens and also facilitates the absorption of essential nutrients by the plant (Beauregard PB 2013.)
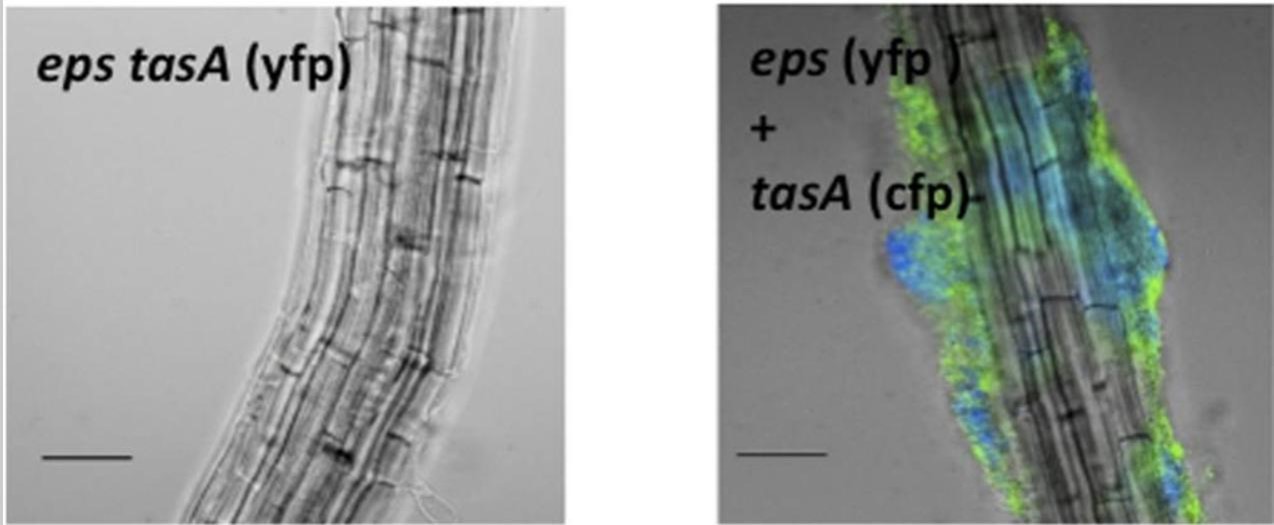
Picture 2: B. subtilis forms biofilms on roots of Brassicaceae A. thaliana. Colonization of the root was observed after 24 h. Credits: Beauregard PB 2013
-
Induced & Acquired resistance #
The application of B.Subtilis primes plants for improved resistance to diseases and pest; it’s a form of plant defense where exposure to specific microorganisms activates earlier and stronger plant’s immune system, thus enhancing its resistance to various imminent of future pathogens.
In other words, when plants are exposed to B. subtilis, they “remember” this experience and are better able to defend themselves.
Bacillus subtilis triggers two different defense pathways in plants: Systemic Acquired resistance (SAR) and Induced Systemic Resistance(ISR).
SAR is triggered by pathogens and involves the production of antimicrobial compounds.
ISR is triggered by beneficial microbes and helps plants respond more quickly to threats.

Picture 3: B. subtilis trials on tomato induces resistance against botrytis cinerea. Credits: Naturnova™ labs
Subtilis acts as a biological control agent against various plant pathogens.
Through the production of antibiotics and antifungal compounds, it inhibits the growth and spread of harmful microorganisms, even by physical attack(osmotic unbalance leading to pathogens cells perforation (Caulier S, 2019) (Ledger EVK, 2022)
By synthesizing dozens of free L-amino acids ready to be used by plants, B. Subtilis promotes growth, effectively fortifying their defenses against pathogens. Such amino acids are organic nutrients that can be directly absorbed by plants, chelate other medium and trace elements, and improve the plant’s utilization of a variety of nutritional elements.
By producing powerful ferric iron–chelating molecules called siderophores to scavenge iron from their environments, B. Subtilis efficiently deplete iron from the environment, making it less available to certain competing microorganisms including plant pathogens (H Kesaulya 2018).
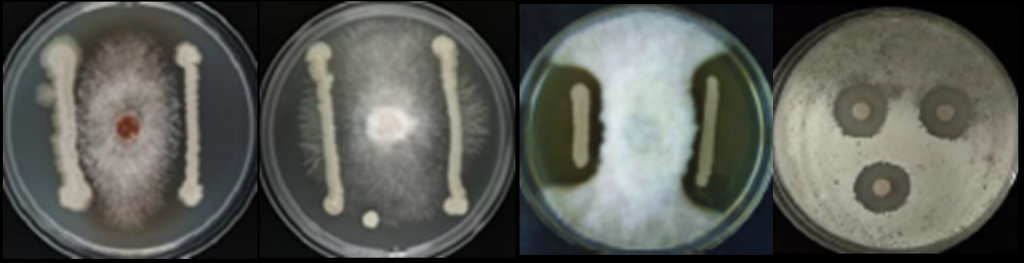
Picture 4: (from left to right) B. Subtilis vs R.Solani,F. Oxysporum, P.Infestans, S Scarb Credits: Naturnova™ labs
These features have significant implications for reducing the reliance on chemical pesticides, thereby lowering the environmental burden.
How does B. Subtilis nourish the crops? #
Except for the compounds involved in the enhancement of crop growth and defense, B. Subtilis also contributes to Phosphorus and Nitrogen availability.
Phosphorus Solubilization
B. subtilis is renowned for its phosphorus-solubilizing abilities while tolerating harsh environments. Through the secretion of organic acids and enzymes, it converts insoluble phosphates into a plant-available form. This, in turn, enhances phosphorus uptake by crops, promoting robust growth (Saeid A, 2018).
Nitrogen Fixation Enhancement
Some strains of B. subtilis bacteria can help plants grow by converting nitrate into ammonia, which is a form of nitrogen that plants can use. This happens when there is no or not enough oxygen in the soil. (Hiremath, 2012)
How does B. Subtilis improve the soil? #
Subtilis aids in the decomposition of organic matter in the soil, thus enriching it with humus. This process not only enhances soil structure but also contributes to long-term soil health and fertility, as it stimulates the growth of beneficial bacteria in the soil, reducing re-cropping obstacles and providing a good environment for root growth. (Kim JK, 2005)
The improvement in soil structure and stability facilitated by B. Subtilis aids in reducing soil erosion and maintaining the integrity of agricultural landscapes.
What emerged from field trials? #
In the Italian 2020 controlled trial, the practical application of B. Subtilis as a biocontrol agent against R. solani, Pythium sp., and Fusarium sp. on maize seedlings was examined. The seeds were subjected to bacterial invasion in enclosed containers. Following the transplantation process, the plants received daily watering. The trials were repeated three times. At the conclusion of the 4-week growth period, the seedlings’ roots were severed at the soil surface.
Pythium sp. was found to induce damping-off both before and after emergence. B. subtilis effectively managed root rot in the maize seedlings. Picture 6 illustrates the dense root system of the maize seedlings after treatment with Bacillus B77, in contrast to the control depicted in picture 6 b. B. Subtilis noticeably regulated the presence of root rot fungi, particularly R. solani and Pythium.
Plant pathogenic microorganisms, such as R. solani, Pythium, and Fusarium, pose a threat to young, emerging plants by causing damping-off. These pathogens are opportunistic and primarily target weak seedlings or germinating seeds. Maize seeds inoculated with R. solani germinated successfully but were subsequently attacked just below the soil surface, leading to the rotting of seedlings’ roots or stems.
B. subtilis has the potential to produce various antibiotics significant for this trial.
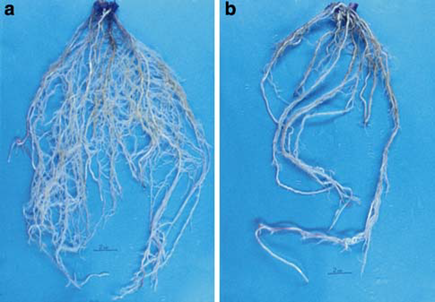
Picture 6: The beneficial effect of B. Subtilis on the damping-off caused by Pythium sp. on maize roots. (a) B. Subtilis + Pythium sp. (b) Pythium sp. only

Picture 7: Effect of Bacillus subtilis 776 and B381 on control of Rhizoctonia solani (RH) R-maize root, ST- seed treatment
In the Mongolian 2022 open field trial of 130 acres, a combination of B.Subtilis with Trichoderma harzianum and Porphyra lilacinus was tested on several varieties of potatoes:
Atlantic., Dutch 15, Black King, Red beauty, Eugene, V6, V7, Jingzhang potato n.2.

Picture 8: potatoes at the beginning of treatment with the bacterial mixture (powder)
All demonstration plots showed no soil-borne diseases such as scab, powdery scab, nematodes, etc., and the overall growth was good.
The mixture showed excellent germination effect after seed dressing, good rooting in the seedling stage, and good resistance to scab and powdery scab at harvest was witnessed. No nematode disease was found.
During harvesting, traces of the Microbial mixture were observed on the potato pieces. When the seed potatoes samples were mailed back to Naturnova™ labs for testing and sequencing, the gene with the same sequence of Trichoderma harzianum was found, further confirming Trichoderma harzianum long-lasting effect

The measured yield of Alantic type potatoes was 19. 352,75 kg/ha (control 18.223,64kg/ha), Eugene potatoes 28.672,3kg/ha (control 27.177,63 kg/ha); Dutch 15 potatoes 22.099,45kg/ha (control 20.803,60 kg/ha). All varieties showed higher yields than the control plots.
Conclusion #
It’s important to note that research in this field is ongoing, and the specific strains and application methods of B. Subtilis may vary depending on crop types, soil conditions, and regional factors. Nevertheless its adoption represents a promising stride towards sustainable and eco-friendly farming practices. Its multifaceted contributions span from natural disease control to nutrient management, soil health and synthetic inputs reduction. By harnessing the power of this remarkable bacterium, farmers can unlock the full potential of their crops while contributing to a more environmentally responsible and sustainable agricultural chain. As we move forward in the pursuit of sustainable agriculture, Bacillus subtilis undoubtedly holds a prominent place in shaping the future of farming.
References
Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):E1621-30. doi: 10.1073/pnas.1218984110. Epub 2013 Apr 8. PMID: 23569226; PMCID: PMC3637697.
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front Microbiol. 2019 Feb 26;10:302. doi: 10.3389/fmicb.2019.00302. PMID: 30873135; PMCID: PMC6401651.
Errington J, Aart LTV. Microbe Profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse. Microbiology (Reading). 2020 May;166(5):425-427. doi: 10.1099/mic.0.000922. Epub 2020 May 4. PMID: 32391747; PMCID: PMC7376258.
Hiremath, Gurusiddesh. (2012). ISOLATION AND CHARACTERIZATION OF NITROGEN FIXING BACILLUS SUBTILIS STRAIN AS-4 FROM AGRICULTURAL SOIL. International Journal of Recent Scientific Research. 3. 762-765.
H Kesaulya et al 2018 IOP Conf. Ser.: Earth Environ. Sci. 102 012016
Ji SH, Gururani MA, Chun SC. Expression analysis of rice pathogenesis-related proteins involved in stress response and endophytic colonization properties of gfp-tagged Bacillus subtilis CB-R05. Appl Biochem Biotechnol. 2014 Sep;174(1):231-41. doi: 10.1007/s12010-014-1047-3. Epub 2014 Jul 24. PMID: 25055794.
Kim JK, Mulrooney SB, Hausinger RP. Biosynthesis of active Bacillus subtilis urease in the absence of known urease accessory proteins. J Bacteriol. 2005 Oct;187(20):7150-4. doi: 10.1128/JB.187.20.7150-7154.2005. PMID: 16199586; PMCID: PMC1251626.
Ledger EVK, Sabnis A, Edwards AM. Polymyxin and lipopeptide antibiotics: membrane-targeting drugs of last resort. Microbiology (Reading). 2022 Feb;168(2):001136. doi: 10.1099/mic.0.001136. PMID: 35118938; PMCID: PMC8941995.
Vlamakis, H., Chai, Y., Beauregard, P. et al. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11, 157–168 (2013). https://doi.org/10.1038/nrmicro2960
Saeid A, Prochownik E, Dobrowolska-Iwanek J. Phosphorus Solubilization by Bacillus Species. Molecules. 2018 Nov 6;23(11):2897. doi: 10.3390/molecules23112897. PMID: 30404208; PMCID: PMC6278551.
Zhou C, Zhu J, Qian N, Guo J, Yan C. Bacillus subtilis SL18r Induces Tomato Resistance Against Botrytis cinerea, Involving Activation of Long Non-coding RNA, MSTRG18363, to Decoy miR1918. Front Plant Sci. 2021 Feb 3;11:634819. doi: 10.3389/fpls.2020.634819. PMID: 33613592; PMCID: PMC7887324.





